Objective: The objective of this study was to investigate the efficacy and safety of venetoclax (VEN) in combination with demethylating agents (HMAs) among newly-diagnosed acute myeloid leukemia (AML) who have fatal comorbidities.
Methods: The study conducted a retrospective analysis of 16 patients with severe complications of primary acute myeloid leukemia (AML) who were admitted to the Hematology Intensive Care Unit (H-ICU) of the First Affiliated Hospital of Soochow University between January 1, 2021 and January 31, 2023. These patients were treated with the VEN+HMAs regimen. The study analyzed the remission rate, overall survival (OS) rate, and the incidence of adverse events.
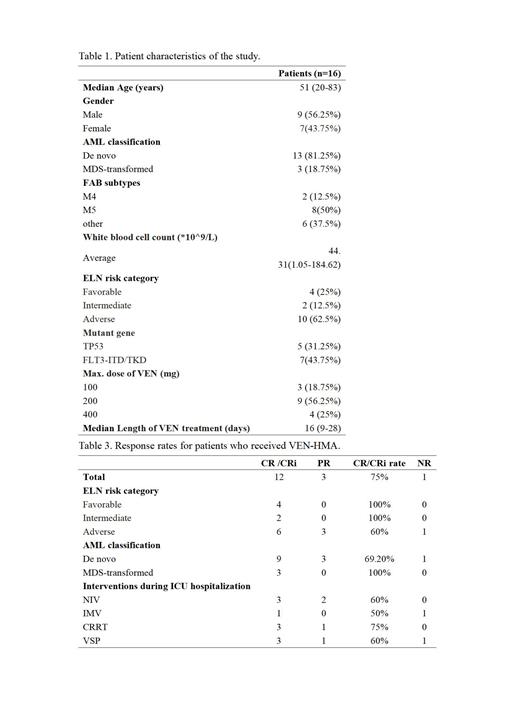
Results. The study included sixteen patients diagnosed with primary unfit-AML who were admitted to H-ICU. The median age was 51 years (range: 20-83), and 9 of them were male (56.2%). The mean APACHE II score was 18.9, the mean SOFA score was 6.2. The average number of days spent in the ICU was 27.3 days. Among them, 15 patients had coexisting pulmonary infections, 5 were on noninvasive ventilators, 2 were on invasive ventilators, and 5 were receiving vasoactive drugs. Additionally, 2 patients had pulmonary hemorrhage, 2 had cerebral hemorrhage, and 4 had severe tumor lysis syndrome and received CRRT treatment. Among the patients, 13 (81.25%) had primary AML, 3 (18.75%) had MDS-transformation, 4 were classified as low-risk, 2 as intermediate-risk, and 10 as high-risk. Decitabine was used in 3 patients, azacitidine in 13 patients, VEN at a maximal dose of 100 mg in 3 patients, 200 mg in 9 patients, and 400 mg in 4 patients. The median duration of venetoclax administration was 16 days. After first course of VEN+HMAs, 12 cases (75%) achieved CR/CRi, and 3 cases (18.75%) achieved partial remission (PR), resulting in an overall response rate (ORR) of 93.75%. Among the patients with TP53 mutation (5 cases), 2 achieved CR and 2 achieved PR, with an ORR of 90%. Additionally, there were 8 cases of AML-M5, of which 6 achieved CR/CRi and 2 achieved PR, with an ORR of 100%. The ORR rate was 50% in patients with invasive ventilators and 100% in patients with CRRT. The average duration of granulopathy was 23 days. All patients experienced grade 3-4 hematological adverse events (AE), with an infection incidence of 44.4% during the period of granulopathy. Among these infections, 3 cases were caused by Acinetobacter baumannii, and 1 case was caused by Klebsiella pneumoniae. There were two cases of early mortality due to cerebral hemorrhage and septic shock respectively. With a median follow-up of 9.5 months (range: 0.5-23), the overall survival (OS) rate was 43.75%, with OS rates of 83.3% and 20% in the low- and high-risk groups, respectively.
Discussion: The combination of VEN+HMAs resulted in a high therapeutic response rate among newly-diagnosed AML patients with severe complications. It was found to be safe, well-tolerated, and associated with a high remission rate using reduced dosage and dosing regimen.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal